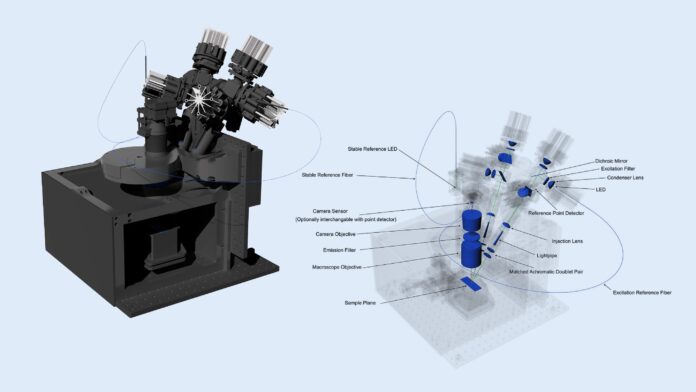
A groundbreaking open-source instrument developed by European researchers promises to revolutionize fluorescence and electroluminescence imaging, democratizing access to cutting-edge techniques across various scientific disciplines. This new luminescence macroscope, detailed in the journal Optics Express, offers a flexible, affordable alternative to expensive, custom-built lab setups often required for advanced light microscopy.
The innovative device, supported by the DREAM project, seamlessly blends affordability with precision and versatility. Unlike conventional imaging instruments confined by rigid optical designs, this “macroscope” empowers researchers to program intricate illumination sequences and precisely control multiple wavelengths, enabling high-speed imaging and time-resolved analysis. Importantly, it accommodates a diverse range of sample types, from delicate plant tissues to complex optoelectronic devices.
Breaking Down Barriers in Luminescence Imaging
Luminescence imaging has become indispensable in modern science, revealing the hidden workings of molecules and living organisms invisible to the naked eye. However, implementing sophisticated illumination protocols often requires specialized knowledge in optics, electronics, and software development—a barrier for many researchers.
“Our aim was to eliminate that hurdle,” explained Dr. Ian Coghill of École Normale Supérieure, Paris, a co-lead author on the study. “We’ve created a system that anyone can easily replicate without needing expert training.”
The research team has taken a revolutionary step by providing completely open access to all essential resources: comprehensive CAD files, detailed assembly instructions, calibration protocols, and user-friendly Python-based control software. By utilizing readily available components, much of it 3D-printed, the entire system can be built for under €25,000—a fraction of the cost of comparable commercial setups. This significantly lowers the financial barrier to entry, empowering smaller labs and interdisciplinary teams to pursue research previously accessible only to large institutions with extensive resources.
A Versatile Tool Across Diverse Fields
The macroscope’s modular design accommodates a wide range of illumination sources spanning ultraviolet to near-infrared wavelengths (405–740 nm) and enables synchronized imaging at speeds up to 100 frames per second. Researchers can tailor the light modulation sequences—using sinusoidal, pulsed patterns, or custom designs—to precisely probe the dynamic behavior of photoactive systems.
The researchers have already demonstrated its broad applicability across various fields:
-
Plant Physiology: Tracking herbicide uptake in thaliana plants and measuring photosynthetic activity using dynamic fluorescence techniques.
-
Protein Photophysics: Distinguishing distinct types of reversibly photoswitchable fluorescent proteins based on their unique “kinetic fingerprints” through advanced imaging methods like RIOM (Rectified Imaging under Optical Modulation).
-
Optoelectronic Devices: Mapping the frequency-dependent electroluminescence in solar cells and LEDs, shedding light on charge transport and recombination processes within these devices.
“These examples only scratch the surface of what’s possible,” notes Dr. Ludovic Jullien, senior author and coordinator of the DREAM project. “By combining open hardware with programmable illumination, we aim to empower both fundamental research and practical innovation in fields ranging from plant biology and photonics to renewable energy.”
True to its open-science ethos, the DREAM project has made all build files, analysis scripts, and experimental data freely accessible on Zenodo. The researchers encourage the scientific community to adapt, modify, and expand upon this platform.
“This is more than just a prototype,” emphasizes Dr. Coghill. “It’s an open foundation—a stepping stone for anyone to explore the fascinating world of dynamic photophysics.”